ど…様専用です、K18WGシェルカメオ、ブローチ兼ペンダント、専門店より出品です
(税込) 送料込み
商品の説明
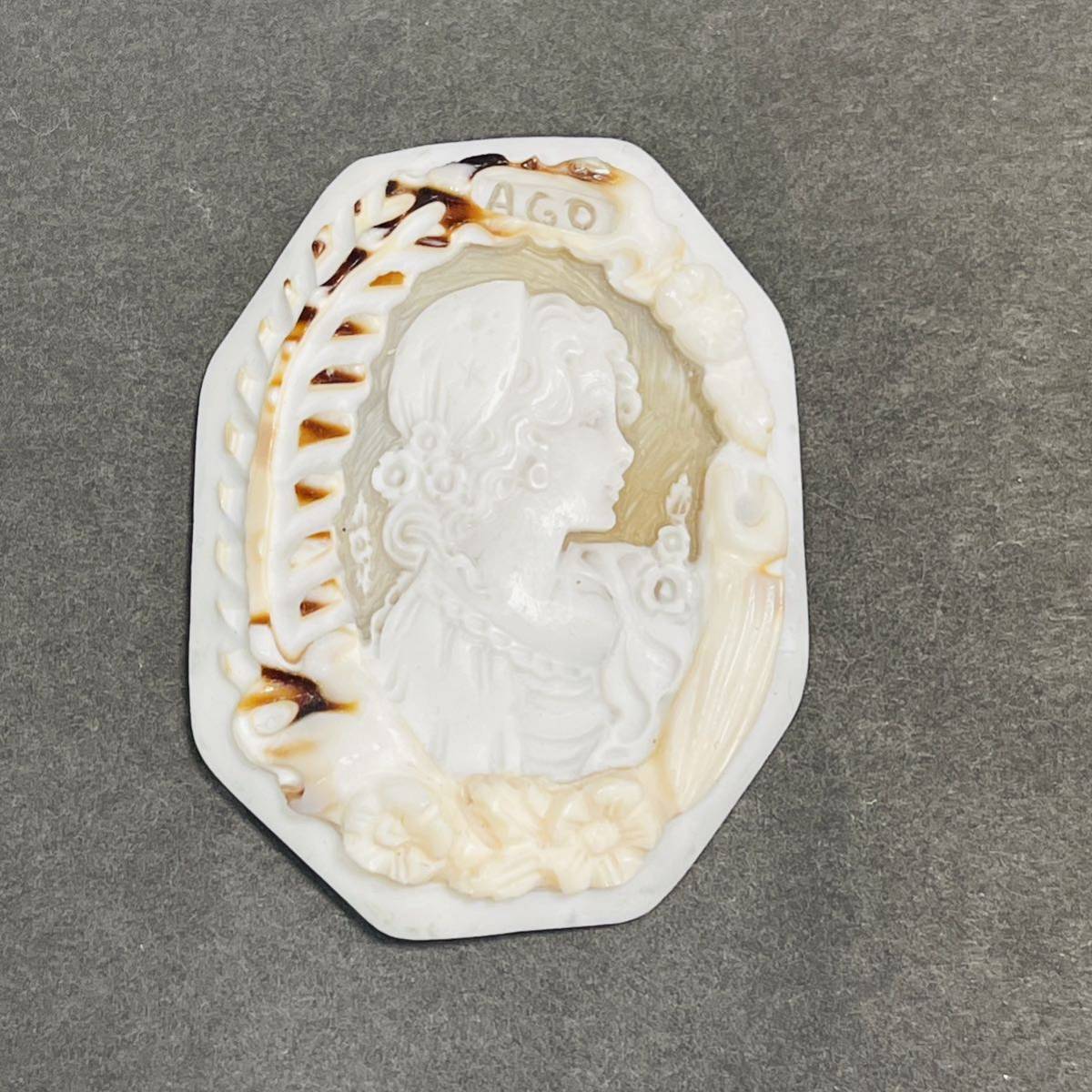
本物のK18WGシェルカメオ、ブローチ兼ペンダント、専門店より出品です。
☆枠材質 K18WG(刻印入り)
☆サイズ 縦約50mm 横約39mm
☆作家 フルーリォ
☆総重量11.8g
※ご覧いただきありがとうございます、こちらの商品は、店売りは、55,の商品です、たいへんお買い得になっておりますので、この機会に是非ご検討宜しくお願い致します。
#イチモト商品の情報
| カテゴリー | レディース > アクセサリー > ブローチ/コサージュ |
|---|---|
| 商品の状態 | 新品、未使用 |

2023年最新】シェルカメオk18枠の人気アイテム - メルカリ

K18 月と少女 大きなシェルカメオブローチ ペンダントトップ兼用

楽天ランキング1位】 ジョバンニ アパ ペンダントトップ ブローチ 585

楽天ランキング1位】 ジョバンニ アパ ペンダントトップ ブローチ 585

K18 月と少女 大きなシェルカメオブローチ ペンダントトップ兼用

楽天ランキング1位】 ジョバンニ アパ ペンダントトップ ブローチ 585

バーゲンで CHANEL シャネル リボン リュバン ネックレス チョーカー

ポイント5倍 ヤフオク! - イタリアカメオ彫刻家フルーリオ作シェ

ポイント5倍 ヤフオク! - イタリアカメオ彫刻家フルーリオ作シェ

バーゲンで CHANEL シャネル リボン リュバン ネックレス チョーカー

24時間以内発送☆大型 カメオ 18金 18K ブローチ 80s ヴィンテージ-

有名人芸能人】 カメオ ブローチ ブローチ兼ペンダント

K18・18金 メノウ メノー ストーンカメオ ブローチ 2way-
高級シェルカメオ16.7g】AGO CAMEO ペンダントトップ カメオ カメオ

ふるさと納税 k18 わんちゃん ペンダントブローチ 5.8g ブローチ

早割クーポン! ディアマナDリミテッド60S ピンスリーブ付 3W用 D

新しいブランド k18 ベビーパール ピンブローチ ブローチ/コサージュ

楽天市場】シェルカメオブローチ兼ペンダントK18/30.3g/W60×H75mm

鑑定済 正規k18カメオブローチペンダントトップ smcint.com

話題の人気 【美品】イヴ・サンローラン カサンドラ ブローチ ブローチ

ポイント5倍 ヤフオク! - イタリアカメオ彫刻家フルーリオ作シェ

2022人気の 【ご専用】MIRACLE ヴィンテージブローチ 他10点 ブローチ

ポイント5倍 ヤフオク! - イタリアカメオ彫刻家フルーリオ作シェ

ダイヤモンドk18wgピアス 限定価格セール! 8160円 www

公式の店舗 ヴィンテージ ブローチ 140点 まとめて まとめ売り 昔の

トリロジー 天然ダイヤピアス 0.5ct K18WG 古典 vidatum.com

値下げ】 Carven カルバン ブローチ 蝶 バタフライ ヴィンテージ

カメオ ブローチ | yasbil.org

ポイント5倍 ヤフオク! - イタリアカメオ彫刻家フルーリオ作シェ

日本限定モデル】 K18wg カメオ K14wg カメオ ブローチ 2WAY

期間限定お試し価格】 K18WG ベネチアンチェーンネックレス 50cm

専用ページ | fecd.org.ec

ブローチ/コサージュ カメオ ブローチ 58 月の女神【刻印サインあり

www.haoming.jp - k14イタリアシェルカメオペンダント&ブローチ2.5g

割引クーポン 石川暢子 NOBUKO ISHIKAWAのブローチ兼ペンダントトップ

華麗 シャネル CHANEL ヴィンテージ 極美品 溶岩 ブローチ ブローチ

qoj.YC704 K18 シェルカメオ 天使と馬 スコニャミリオ作 ブローチ

カメオ ブローチ 兼ペンダントトップ 2WAY K18YG イエローゴールド

SELECT JEWELRY カメオ 三層彫 ブローチ K18WG レディース-www.ecosea.do

有名人芸能人】 カメオ ブローチ ブローチ兼ペンダント






商品の情報
メルカリ安心への取り組み
お金は事務局に支払われ、評価後に振り込まれます
出品者
スピード発送
この出品者は平均24時間以内に発送しています














